Introduction
The history of the dental implant dates back to 3000 B.C, it was the period when the ancient Egyptian civilization prospered. Dental replantation and transplantation was first mentioned by Allen in 1687 and 2 decades later, it started being practiced as a viable treatment option.[1] Dental implants have been used for decades now, but it is has only been a few decades that they have achieved widespread acceptance by the profession. Now days, dental implants are the first choice restorative and prosthetic rehabilitative tool for the replacement of a missing tooth or teeth or even a completely edentulous jaw. It gives the most predictable long-term solution- for an increasing number of patients around the world.[2] Integration of implants with the peri-implant bone is critical for efficient osseointegration and prolonging the longevity of implant supported prosthesis. Osseointegration is a function of a multitude of factors including implant surface characteristics, host factors and loading conditions. The factor we can control the most is the implant surface characteristics we choose for the patient. Although professionals have used oral implants worldwide, but the basic information relating implant surface characteristics, it’s modifications and it’s role in enhancing clinical performance is often lacking. Every year, advances in the microtopography and nanotopography are introduced which influence osseointegration. It is therefore, important to know whether certain surface modifications or particular material will improve clinical success and provide the best available treatment for the lost tooth.
Discussion
Recently growing technology is rapidly advancing the surface engineering aspect in implant dentistry. Such advances have resulted in more complicated surface properties from macro to micro to nanometer scales. Surface topography of an implant can be designed by making porous and/or by coating the implant surface to increase bone-implant contact since the anatomic surface of bone can’t be controlled.
Method for surface modifications of implants are described broadly under three major categories: Physical, mechanical and chemical. These methods are employed to modify the surface characteristics, which include implant surface chemistry, morphology and structure.
The mechanical methods employed are blasting, grinding, machining and polishing. The chemical methods of implant surface modifications include chemical treatment with acid or alkali, sol-gel, hydrogen peroxide treatment, chemical vapor deposition and anodization. Chemical modification of implant surface has shown to enhance the wettability. The physical methods to modify the implant surface are plasma spraying, sputtering and ion deposition.
The methods to increase the surface roughness of the implant can be classified into:
a) Turned surfaces
b) Sandblasted surfaces
c) Acid etched surfaces
d) Sandblasted and acid etched surfaces (SLA)
e) Anodized surfaces
f) Plasma sprayed surfaces – titanium plasma spray
g) Sputter deposition
h) Laser modified surfaces
i) Biomimetic precipitation
j) Bioactive glass coatings
Turned Surfaces
It is the most commonly used method to enhance the surface roughness. After the turning process, the implants are subjected to cleaning, decontamination and sterilization process. These surfaces are also called machined or smooth surfaces. Microscopically turning results in slight roughness because of the grooves and ridges and marks of the tools used during the manufacturing process. The result of turned surface is that, it induces distance osteogenesis, which requires a longer waiting time between surgery and implant loading.
More enhancements were tried and tested to modify the surface from turned to more roughened, which helped improve stabilization of implant by increasing the surface area. The modified surface is further enhanced by additive methods like ceramic coatings, Plasma spraying, etc. or by subtractive methods like Sandblasting and acid etching. Typical Sa values for turned surfaces are 0.3-1.0µm.
Example: LLC implant system. Fig. 1
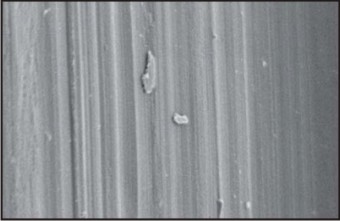 | Fig.1 : Scanning Electron Micrograph Of Machined Implant Surface
 |
Sandblasted Surfaces
The implant roughness can be increased by grit blasting technique using small titania or alumina particles. When the particles hit the implant surface it creates surface defects like craters. The surface roughness is a function of the particle used to blast the implant. It is dependent on the particle material, size, shape, speed and density. As the particle size increases, the surface roughness of the implant increases.
This roughness provides a mechanical interlocking with peri-implant bone. This technique was the first widely accepted, clinically applied surface modification for titanium implants as this showed better osseointegration than the turned surfaces.Typical Sa values are 0.5-2.0 μm. The blasting procedure is performed by using agents such as aluminium oxide and titanium oxide. Sandblasting helps in adhesion, proliferation, and differentiation of osteoblasts. This technique has been further modified by acid etching to increase the surface roughness, commonly known as SLA (sandblasted and acid etched).
Example: MIS implant system Fig.2
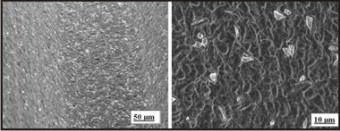 | Fig.2 : Scanning Electron Micrograph Of Sandblasted Implant Surfaces
 |
Acid Etched Surfaces
Etching the implant surface with strong acids results in a pitted surface because of removal if grains, as some phases or impurities are selectively sensitive to etching. Acid etching removes the top oxide layer from the titanium implants. It is a function of acid concentration, the soaking time, acid temperature, the bulk material and the surface microstructure. It results in minimally rough surface as the typical Sa values are 0.3-1.0 μm. Different combinations of HCl, H2SO4, HNO3 and HF are used. Acid etching results in a homogeneous roughness, increased active surface area and better bioadhesion. Higher bone implant integration is seen for acid etched as compared to turned surfaces.
A dual acid-etched technique has been proposed to produce a micron level roughness, which could result in achieving advanced osseointegration. The titanium implants are immersed in a combination of HCl and H2SO4. The combination mixture is heated above 1000C. This technique results in more efficient osteoconductive process, by the attachment of the oesteogenic cells and the fibrin, which further results in bone formation directly on the surface of the implant.
Examples: MIS implant Fig.3
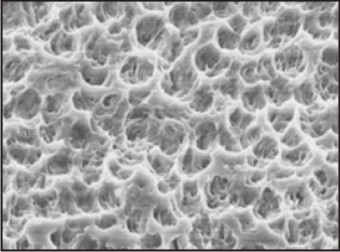 | Fig.3 : Scanning Electron Micrograph Of Acid Etched Titanium Surface
 |
Sandblasted And Acid Etched Surface (Sla)
Dental implants are usually both blasted by particles of large grit size 250-500 μm, followed by etching with acids HCl andH2SO4. Then subsequent etched by acids. Sandblasting results in improved surface roughness and acid etching results in enhanced micro texture and cleaning. This is done to get a dual surface roughness and also removal of embedded blasting particles.
The Sa values for blasted and acid etched implants are 1-2 μm. Acid etching changes the surface texture and creates a layer of titanium hydride, which has a thickness of 1-2 μm between the surface oxide layer and the implant material.
Then, by rinsing the SLA implant in a nitrogen atmosphere and storing in saline medium until surgical placement, the amount of contamination by carbon was less and it helped in improving the hydrophilic nature of the implant surface. The process is carried out to create a new hydrophilic surface called as SLActive (Fig. 4). This process makes the implant surface chemically active that is conditioned to the human body. Additionally, the anions from the acid could be incorporated in the oxide layer such as fluoride ions if etched in hydrofluoric acid (Fig. 5). SLActive implants have greater stability, higher bone contact and reduced healing time as compared to SLA implants.
Example: GENESIS implant system Fig.4 & Fig. 5
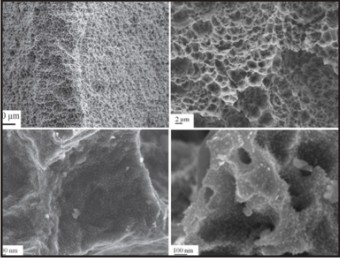 | Fig.4 : Scanning Electron Micrograph Of Sand Blasted And Acid Etched Implant Surface (Sla). Nano Features On The Slactive Implant Surface.
 |
 | Fig.5 : Scanning Electron Micrograph Of Titanium Dental Implants In A Fluoride Modified Surface.
 |
Anodized Surfaces
Anodized surface implants are those which act as anodes in a galvanic cell immersed in a suitable electrolyte. Anodic oxidation results in a thick titanium oxide layer and a porous surface. The oxidation alters the oxide layer and makes it more susceptible to osseointegration. The surface with micropores show enhanced cell proliferation and attachment and increased tissue healing.Sa values are ranging from 0.96 to 1.03µm.[3] Osseointegration in anodized surfaces has been explained by two mechanisms: mechanical interlocking by bone growth in pores and biochemical bonding on the bone-implant interface. The anodization process depends on current density, concentration of acids, composition and electrolyte temperature.
When compared to sandblasted, acid etched or turned surfaces, the anodized surfaces have shown more efficient osseointegration.
Example: GENESIS implant system Fig.6
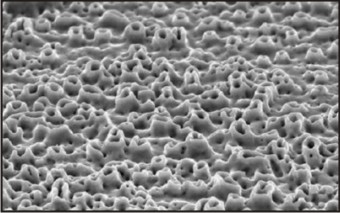 | Fig.6 : Scanning Electron Micrograph Of An Anodized Implant SurfaceFig.6 : Scanning Electron Micrograph Of An Anodized Implant Surface
 |
Plasma-sprayed Surfaces
The use of plasma-sprayed surfaces has been reported in orthopaedics studies since 1970s. Later it was observed that the bone around the dental implants was formed without an intervening layer of connective tissue. Plasma sprayed implants are formed by spraying the molten metal on the titanium base, which results in an implant surface with irregular valleys, troughs, peaks, crevices and pores, increasing the microscopic surface area by 6-10 times. Plasma spraying is the most commonly used technique for depositing calcium phosphate coatings to improve the implant’s interaction with the bone.
The major advantage of this technique is that these coatings give the implant a micro-porous structure on which bone grows more readily. Osseointegration has shown to be the most efficient when compared to other surface modifications.
The titanium powder is injected into a plasma torch at a very high temperature that partially melts the powder. These particles are then sprayed on to the implant surface, where they condense and fuse together to form a film. This method uses a carrier gas, which ionizes the plasma and superheats the titanium particles and propels it towards the implant surface to be coated. This forms a three dimensional surface, which simulates adhesion osteogenesis.
Bio-ceramic coatings (plasma sprayed hydroxyapatite coatings) have gained considerable attention due to their natural bone like components. Histologically, the cortical bone reaction to the titanium and HA coated titanium was similar; the only difference was in the amount of bone deposition along the non-cortical bone. HA coated implant surface induced more bone deposition then the uncoated implant surface.
Plasma sprayed surfaces obtained with more reactive materials have been proposed to accelerate and enhance the bone ingrowth into pores of the implant surface. An alkali modification after plasma spraying, using sodium hydroxide solutions at 400C for 24 hours has been proposed. Few disadvantages of using plasma sprayed implants is the detachment of titanium after implant insertion, non uniform thickness of the deposited layer and relative variable composition of coating.
Example: BIOMET 3i implant system Fig 7
 | Fig 7 : Scanning Electron Micrograph Of Plasma Sprayed Surface At The Junction With Polished Collar.
 |
Sputter Deposition
In this technique, the target material is bombarded with high-energy ions, which propel atoms or molecules from the target into the vacuum chamber, which are deposited on the titanium implant.
This technique is specifically useful to deposit bioceramic (CaP) films because this technique provides greater control of coating thickness and enhanced adhesion between the implants and the coating. The hydroxyapatite coating varies in the thickness from 0.5-3.0 μm.[4] The drawback is that the rate of deposition of particle is slow. This drawback is overcome by using Radiofrequency Magnetron Sputtering (magnetically enhanced variant of diode sputtering). Fig.8.
Examples: Genesis Implant System, Biohorizon Implant System, Hiossen Implant System, Kat Implant System
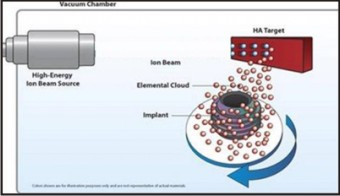 | Fig.8 : Ion Sputtering
 |
Laser Modification
Laser is the latest technology to analyze a three dimensional structure at micron level. It is especially suitable for complex geometries. A short pulse of single wavelength light, focused on one spot, modifies the surfaces and generates high-resolution complex features.[5] The major benefit of laser modification is that it can be used selectively on the valley and some parts of the flank of the implant thread. The remaining part of the implant surface can remain turned. Since the flank of the implant thread is more prone to plaque accumulation, therefore, it is advised to keep the flank surface relatively smooth, to decrease the chances of peri-implantitis. The remaining part of the implant can have a rough surface to increase the surface area for osseointegration.
Example: BIOHORIZONS IMPLANT SYSTEM Fig.9
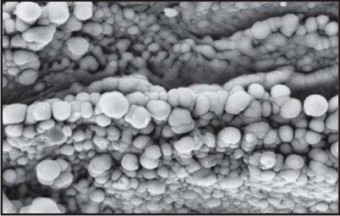 | Fig.9 : Scanning Electron Micrograph Of Laser Modified Implant Surface
 |
Biomimetic Precipitation
Kokubo and workers (1990) developed the biomimetic deposition of Calcium phosphate on the surface of implant material. In this method, the hydroxyapatite is coated on the implant surface in a simulated body fluid (SBF) under optimum physiological temperature and pH. It is should be immersed for 14-28 days with the intermittent replenishment of SBF. The thickness of hydroxyapatite layer is directly proportional to the immersion duration. The layer of Hydroxyapetite is usually rough and porous, facilitating early bone in-growth into the pores.
Recently Strontium ions have been added to the hydroxyapatite, which changes the topography from plate like flat to sphere like round morphology. The Strontium bonded chemically and replaced calcium from hydroxyapatite. The advantage of adding Strontium was that it made the implant bioactive and promoted early bone formation. Fig.10
 | Fig.10 : Scanning Electron Micrograph Of Biomimetic Precipitation On The Titanium Implant Surface
 |
Bioactive Glass Coating
Hench introduced the silica based bioactive glass (1971) which is a slowly resorbing osteoconductive material and it was able to form a chemical bond with the bone. Recently, bioactive glass coatings were prepared on titanium and 4Ti6Al4V. This ground coating of glass can be enriched with other surface reactive glass coatings like embedded hydroxyapatite, reactive plasma spraying and sol-gel derived silica coating.
Titanium implants coated with bioactive glass showed improved osseointegration without the formation of an intervening connective tissue capsule. These implants have higher bone-implant contact and also require higher removal torque when compared to uncoated titanium implants. Fig.11
 | Fig.11 : Scanning Electron Micrograph Of Bioactive Glass Coated Implant
 |
Conclusion
A multitude of factors govern the osseointegration, making it difficult to predict the implant’s success. The longevity of implant is strongly affected by the surface characteristics. Roughened surfaces have better biomechanical stability and favours osseointegration.[6]
Furthur development in the implant dentistry would be required to modify the interfacial chemistry between bone and tissue interface at cellular level. Biological mediators of growth and differentiation at the bone-implant junction may enhance tissue healing.
Recently clinical studies on antibiotic impregnated implants are being done to prevent surgical site infection. Surface modifications like biomimetic precipitation have improved the biological response to the implant surface manifold.
References
1. Allen C. Curious observations on the teeth. London. John Bale, Sons & Danielsson, LTD 1687
2. Haswell M. Dental implants: a different perspective, part one. Implant practice, 2009;2:44-57
3. Misch CE, Contemporary implant dentistry 3rd edition, Moseby (Mussouri) 2008.
4. Puleo DA, Thomas MV. Implant surfaces. Dent Clin N Am 2006;50:323-38.
5. Ahmed MB. Dental implant surfaces – physiochemical properties, biological performance and trends. Implant Dentistry- A Rapidly Evolving Practice2011;pg 19-56
6. Sabene AV. Surface characteristics of dental implants. J Indian Acad Dent Spec Res 2011;2:18-21
|